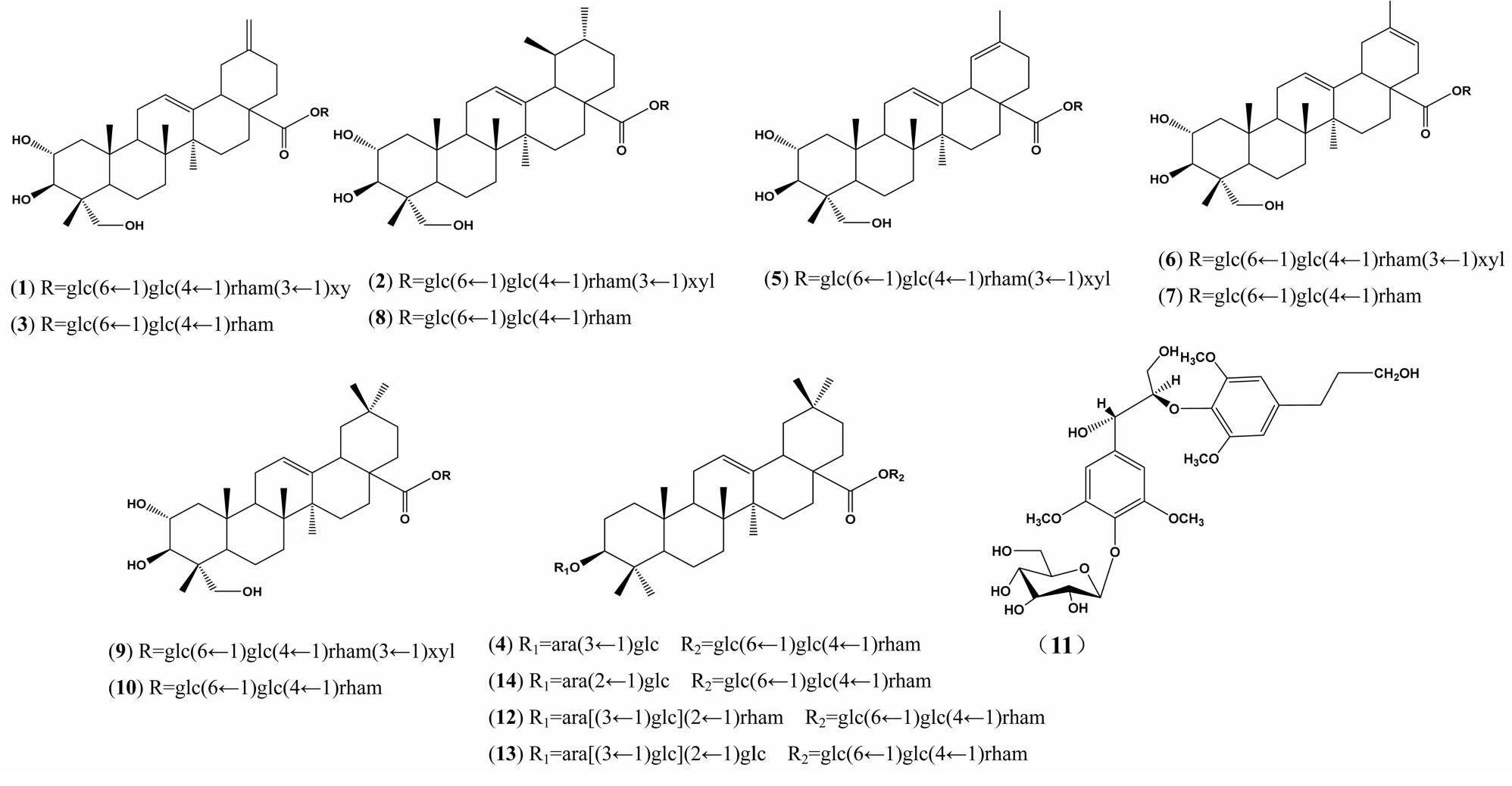
三叶木通是木通科木通属藤本植物,原产于中国和日本,在中国主要分布于陕西与湖北西部、河南南部及甘肃、四川等地[1-2],为2015年版《中国药典》收录的3个木通属植物之一,具有利尿通淋、清火除烦、通经下乳的功效,用于治疗心烦尿赤、水肿、淋症、经闭乳少等症状[3-4]。三叶木通化学成分丰富复杂,包括三萜类、三萜皂苷类、木脂素类、苯丙素类和酚醇苷类等[5],三萜皂苷类为其主要化学成分,现研究表明三萜皂苷在利尿抗水肿、抗菌消炎、抑制肿瘤细胞生长等多方面均有潜在的开发应用价值[6-8],为更好地开发利用该植物药用资源,本课题组对三叶木通进行较为系统的化学成分研究。采用三叶木通75%乙醇提取物通过硅胶柱色谱、凝胶、高压制备技术分离鉴定了14个化合物。分别为木通皂苷B(1),木通皂苷C(2),皂苷PH(3),卵叶银莲花苷A(4),2α,3β,23-三羟基齐墩果烷-30-去甲基-12,19-双烯-28-O-β-D-吡喃木糖-(1→3)-α-L-吡喃鼠李糖-(1→4)-β-D-吡喃葡萄糖-(1→6)-β-D-吡喃葡萄糖苷(5),akemisaponins D(6),akemisaponins E(7),积雪草苷(8),皂苷PJ1(9),scheffoleoside A(10),symplocosneolignan A(11),刺楸皂苷D(12),leonticin E(13),ciwujianoside A1(14)。化合物1~4,11,13,14均为首次从该植物中分离得到。结构式见图1。
1 材料
1260型高效液相色谱仪,1200型制备高效液相色谱仪(美国安捷伦公司);中压液相制备色谱仪(瑞士步琪公司);BP211D型电子天平(德国赛托利斯集团);SB-1000型旋转蒸发仪(日本EYELA公司);电热恒温水浴锅(上海跃进医疗器械厂);UNITY INOVA 600型超导核磁共振仪(美国Varian公司),ZabSpec型质谱仪(美国Micromass公司);C18反相填料(日本YMC公司),柱色谱硅胶、薄层色谱硅胶(青岛海洋化工厂),其他所用试剂均为分析纯或色谱纯。
三叶木通药材由江西南昌济生制药厂提供,经江西省药品检验检测研究院周国平主任中药师鉴定为木通科木通属植物三叶木通Akebia trifoliata的干燥藤茎。标本保存在江西省药品检验检测研究院中药室标本馆。
2 提取与分离
三叶木通药材(100 kg),粉碎后用75%乙醇回流提取3次,每次2 h,提取液合并减压浓缩至无醇味,得干浸膏20 kg。然后加适量水使成混悬液,依次用等体积的二氯甲烷、乙酸乙酯、正丁醇分别萃取2次,减压浓缩至干,得正丁醇部分3 kg。
取正丁醇部分2 kg上D101大孔树脂,分别用10%,30%,50%,70%,95%乙醇梯度洗脱。收集30%乙醇洗脱液,减压浓缩至干,称质量555 g,该馏分样品经硅胶柱色谱(100~200目),以三氯甲烷-甲醇(2∶1)洗脱,经薄层色谱检视,合并相近的组分,得馏分F1~F16。馏分F12(95 g)经硅胶柱色谱,以三氯甲烷-甲醇(5∶1~1∶1)梯度洗脱,得到馏分F12-1~F12-8,F12-1(700 mg)经LH-20型羟丙基葡聚糖凝胶柱(Sephadex LH-20)得馏分(F12-1-1~F12-1-4),F12-1-2(42 mg)经反相高效制备液相色谱,乙腈-水(14∶86)为流动相,得化合物4(5 mg),10(7 mg),F12-4(10 g)过C18中压柱,以乙腈-水(25∶75~29∶71)梯度洗脱,经高效液相色谱检测后,合并为4个部分(F12-4-1~F12-4-4)。F12-4-2(200 mg)经反相高效制备液相色谱,以乙腈-水(21∶79)为流动相,得化合物3(60 mg),F12-5过C18中压柱,乙腈-水(21∶79~23∶77)梯度洗脱,经高效液相色谱检测后,合并为7个部分(F12-5-1~F12-5-7)。F12-5-2(127 mg)经反相高效制备液相色谱,以乙腈-水(18∶82)为流动相,得化合物12(18 mg),13(13 mg)。F12-5-3(2.81 g)经反相高效制备液相色谱,以乙腈-水(21∶79)为流动相,得化合物1(26 mg),5(13 mg),6(37 mg),7(9 mg),9(24 mg)。F16-5-5(639 mg)经反相高效制备液相色谱,以乙腈-水(22∶78)为流动相,得化合物2(86 mg),8(34 mg)。F12-5-6(160 mg)经反相高效制备液相色谱,以乙腈-水(29∶71)为流动相,得化合物11(24 mg)。F12-4(10 g)过C18中压柱,以乙腈-水(25∶75~29∶71)梯度洗脱,经高效液相色谱检测后,合并为2个部分(F12-4-1~F12-4-2)。F12-4-2(56 mg)经反相高效制备液相色谱,以乙腈-水(22∶78)为流动相,得化合物14(18 mg)。
3 结构鉴定
化合物1 白色无定形粉末(甲醇)。ESI-MS m/z 1 097[M+Na]+。1H-NMR(600 MHz,C5D5N) δ:6.22(1H,d,J=7.8 Hz,glc-H-1),5.91(1H,br s,rham-H-1),5.44(1H,br s,H-12),5.27(1H,d,J=7.8,1.8 Hz,xyl-H-1),4.95(1H,d,J=7.8 Hz,glc′-H-1),4.71(1H,s,H-29a),4.66(1H,s,H-29b),4.22(1H,d,J=10.8 Hz,H-23a),3.72(1H,d,J=10.8 Hz,H-23b),1.68(3H,d,J=6.0 Hz,rham-H-6),1.14(3H,s,H-26),1.13(3H,s,H-27),1.12(3H,s,H-25),1.09(3H,s,H-24);13C-NMR(150 MHz,C5D5N) δ:48.0(C-1),69.3(C-2),78.4(C-3),44.1(C-4),48.3(C-5),19.0(C-6),32.3(C-7),40.5(C-8),48.6(C-9),38.9(C-10),24.4(C-11),123.0(C-12),144.0(C-13),42.6(C-14),28.7(C-15),24.0(C-16),48.3(C-17),47.8(C-18),42.1(C-19),148.8(C-20),30.5(C-21),38.1(C-22),66.8(C-23),14.9(C-24),18.0(C-25),18.1(C-26),26.5(C-27),176.3 (C-28),107.8 (C-29),96.2(glc-C-1),74.3(glc-C-2),78.9(glc-C-3),71.5(glc-C-4),78.4(glc-C-5),69.8(glc-C-6),105.4(glc′-C-1),75.9(glc′-C-2),76.8(glc′-C-3),77.7(glc′-C-4),77.7(glc′-C-5),61.7(glc′-C-6),102.8(rham-C-1),72.6(rham-C-2),83.8(rham-C-3),73.4(rham-C-4),70.3(rham-C-5),18.9(rham-C-6),107.9(xyl-C-1),76.1(xyl-C-2),79.1(xyl-C-3),71.3(xyl-C-4),67.8(xyl-C-5)。以上数据与文献[9]报道的数据一致,因此鉴定化合物1为木通皂苷B。
化合物2 白色无定形粉末(甲醇)。ESI-MS m/z 1 113 [M + Na]+。1H-NMR(600 MHz,C5D5N) δ:6.22(1H,d,J=7.8 Hz,glc-H-1),5.91(1H,br s,rham-H-1),5.44(1H,br s,H-12),5.27(1H,d,J=7.8,1.8 Hz,xyl-H-1),4.95(1H,d,J=7.8 Hz,glc′-H-1),4.22(1H,d,J=10.8 Hz,H-23a),3.72(1H,d,J=10.8 Hz,H-23b),1.68(3H,d,J=6.0 Hz,rham-H-6),1.20(3H,s,H-26),1.14(3H,s,H-25),1.09(3H,s,H-27),1.08(3H,s,H-24),0.92(3H,d,J=6.0 Hz,H-29),0.88(3H,d,J=6.0 Hz,H-30);13C-NMR(150 MHz,C5D5N) δ:48.1(C-1),69.0(C-2),78.2(C-3),43.6(C-4),47.9(C-5),18.5(C-6),33.2(C-7),40.2(C-8),48.2(C-9),38.3(C-10),23.8(C-11),126.0(C-12),138.6(C-13),42.5(C-14),28.6(C-15),24.5(C-16),48.3(C-17),53.2(C-18),39.3(C-19),39.1(C-20),36.7(C-21),30.8(C-22),66.5(C-23),14.4(C-24),17.7(C-25),17.8 (C-26),23.7(C-27),176.3(C-28),17.4 (C-29),21.2(C-30),95.6(glc-C-1),73.8(glc-C-2),78.9(glc-C-3),71.1(glc-C-4),77.8(glc-C-5),69.6(glc-C-6),105.2(glc′-C-1),75.5(glc′-C-2),76.4(glc′-C-3),77.2(glc′-C-4),77.2(glc′-C-5),61.3(glc′-C-6),102.1(rham-C-1),72.2(rham-C-2),83.5(rham-C-3),73.0(rham-C-4),70.0(rham-C-5),18.4(rham-C-6),107.5(xyl-C-1),75.7(xyl-C-2),78.4(xyl-C-3),71.1(xyl-C-4),67.3(xyl-C-5)。以上数据与文献[9]报道的数据一致,因此鉴定化合物2为木通皂苷C。
化合物3 白色无定形粉末(甲醇)。ESI-MS m/z 947 [M + Na]+。1H-NMR(600 MHz,C5D5N)δ:6.21(1H,d,J=7.8 Hz,glc-H-1),5.90(1H,br s,rham-H-1),5.44(1H,br s,H-12),4.94(1H,d,J= 7.8 Hz,glc′-H-1),4.70(1H,s,H-29a),4.66(1H,s,H-29b),4.22(1H,d,J=10.8 Hz,H-23a),3.72(1H,d,J=10.8 Hz,H-23b),1.68(3H,d,J=6.0 Hz,rham-H-6),1.15(3H,s,H-26),1.13(3H,s,H-27),1.11(3H,s,H-25),1.10(3H,s,H-24);13C-NMR (150 MHz,C5D5N)δ:48.0(C-1),69.4(C-2),78.6(C-3),44.1(C-4),48.3(C-5),19.0(C-6),32.3(C-7),40.5(C-8),48.6(C-9),38.9(C-10),24.4(C-11),123.0(C-12),144.0(C-13),42.6(C-14),28.7(C-15),24.0(C-16),48.3(C-17),47.8(C-18),42.1(C-19),148.8(C-20),30.5(C-21),38.1(C-22),66.8(C-23),14.9(C-24),18.0(C-25),18.1(C-26),26.5(C-27),176.3(C-28),107.9(C-29),96.2(glc-C-1),74.3(glc-C-2),79.1(glc-C-3),71.5(glc-C-4),78.6(glc-C-5),69.8(glc-C-6),105.4(glc′-C-1),75.9(glc′-C-2),77.0(glc′-C-3),78.4(glc′-C-4),77.6(glc′-C-5),61.7(glc′-C-6),102.8(rham-C-1),73.1(rham-C-2),73.2(rham-C-3),74.4(rham-C-4),70.3(rham-C-5),18.8(rham-C-6)。以上数据与文献[9]报道的数据一致,因此鉴定化合物3为皂苷PH。
化合物4 白色无定形粉末(甲醇)。ESI-MS m/z 1 097 [M+Na]+。1H-NMR(600 MHz,C5D5N)δ:6.26(1H,d,J=7.8 Hz,glc-H-1),5.88(1H,br s,rham-H-1),5.42(1H,br s,H-12),5.40(1H,d,J=7.8 Hz,glc″-H-1),5.21(1H,br s,H-21),5.00(1H,d,J=7.8 Hz,glc′-H-1),4.73(1H,d,J=7.2 Hz,ara-H-1),3.36(1H,dd,J=4.8,12.0 Hz,H-3),1.71(3H,d,J=6.0 Hz,rham-H-6),1.31(3H,s,H-30),1.26(3H,s,H-29),1.11(3H,s,H-27),1.00(3H,s,H-26),0.91(3H,s,H-25),0.90(3H,s,H-24),0.89(3H,s,H-23);13C-NMR(150 MHz,C5D5N)δ:39.1(C-1),27.0(C-2),89.0(C-3),40.0(C-4),56.2(C-5),18.8(C-6),33.5(C-7),40.2(C-8),48.4(C-9),37.4(C-10),24(C-11),123.2(C-12),144.4(C-13),42.4(C-14),28.6(C-15),23.7(C-16),47.4(C-17),42.0(C-18),46.5(C-19),31.1(C-20),34.3(C-21),32.9(C-22),28.4(C-23),17.3(C-24),15.9(C-25),17.8(C-26),26.4(C-27),176.8(C-28),33.5(C-29),24.1(C-30),107.7(ara-C-1),71.9(ara-C-2),84.4(ara-C-3),69.5(ara-C-4),67.3(ara-C-5),106.7(glc-C-1),76.1(glc-C-2),78.5(glc-C-3),72.2(glc-C-4),78.7(glc-C-5),62.9(glc-C-6),96.0(glc′-C-1),74.2(glc′-C-2),79.0(glc′-C-3),71.2(glc′-C-4),78.4(glc′-C-5),69.6(glc′-C-6),105.2(glc″-C-1),75.7(glc″-C-2),76.8(glc″-C-3),79.1(glc″-C-4),77.5(glc″-C-5),61.6(glc″-C-6),103.0(rham-C-1),72.9(rham-C-2),72.8(rham-C-3),74.3(rham-C-4),70.6(rham-C-5),18.8(rham-C-6)。以上数据与文献[10]报道的数据一致,因此鉴定化合物4为卵叶银莲花苷A。
化合物5 白色无定形粉末(甲醇)。ESI-MS m/z 1 097 [M+Na]+。1H-NMR(600 MHz,C5D5N)δ:6.30(1H,d,J=7.8 Hz,glc-H-1),5.91(1H,br s,rham-H-1),5.55(1H,br s,H-12),5.26(1H,d,J=7.8 Hz,xyl-H-1),5.19(1H,br s,H-19),4.93(1H,d,J=7.8 Hz,glc′-H-1),4.23(1H,d,J=10.2 Hz,H-23a),3.71(1H,d,J=10.2 Hz,H-23b),1.69(3H,d,J=6.0 Hz,rham-H-6),1.57(3H,s,H-29),1.16(3H,s,H-26),1.13(3H,s,H-25),1.09(3H,s,H-27),1.07(3H,s,H-24);13C-NMR(150 MHz,C5D5N)δ:48.0(C-1),69.0(C-2),78.3(C-3),44.0(C-4),48.0(C-5),18.4(C-6),33.4(C-7),39.7(C-8),48.1(C-9),38.5(C-10),23.8(C-11),123.5(C-12),142.7(C-13),43.2(C-14),28(C-15),22.9(C-16),45.9(C-17),46.0(C-18),129.5(C-19),129.7 (C-20),26.2(C-21),32.6(C-22),66.4(C-23),14.6(C-24),17.8(C-25),17.9 (C-26),23.6(C-27),176.3 (C-28),23.2 (C-29),95.8(glc-C-1),73.3(glc-C-2),78.7(glc-C-3),70.7(glc-C-4),77.9(glc-C-5),69.4(glc-C-6),105.2(glc′-C-1),75.5(glc′-C-2),76.4(glc′-C-3),78.5(glc′-C-4),77.2(glc′-C-5),61.2(glc′-C-6),102.4(rham-C-1),72.2(rham-C-2),83.2(rham-C-3),73(rham-C-4),70.1(rham-C-5),18.5(rham-C-6),107.4(xyl-C-1),75.4(xyl-C-2),77.2(xyl-C-3),71.1(xyl-C-4),67.3(xyl-C-5)。以上数据与文献[11]报道的数据一致,因此鉴定化合物5为2α,3β,23-三羟基齐墩果烷-30-去甲基-12,19-双烯-28-O-β-D-吡喃木糖-(1→3)-α-L-吡喃鼠李糖-(1→4)-β-D-吡喃葡萄糖-(1→6)-β-D-吡喃葡萄糖苷。
化合物6 白色无定形粉末(甲醇)。ESI-MS m/z 1 097[M+Na]+。1H-NMR(600 MHz,C5D5N)δ:6.23(1H,d,J=7.8 Hz,glc-H-1),5.90(1H,br s,rham-H-1),5.44(1H,br s,H-12),5.26(1H,d,J=7.8 Hz,xyl-H-1),5.21(1H,br s,H-21),4.94(1H,d,J=7.8 Hz,glc′-H-1),4.17(1H,m,H-23a),3.71(1H,d,J=10.2 Hz,H-23b),1.68(3H,d,J=6.0 Hz,rham-H-6),1.60(3H,s,H-29),1.20(3H,s,H-27),1.16(3H,s,H-26),1.13(3H,s,H-25),1.09(3H,s,H-24);13C-NMR(150 MHz,C5D5N)δ:47.8(C-1),69.0(C-2),78.4(C-3),43.8(C-4),48.1(C-5),18.8(C-6),32.9(C-7),40.1(C-8),48.3(C-9),38.5(C-10),24.1(C-11),123.1(C-12),143.8(C-13),42.2(C-14),28.7(C-15),26(C-16),45.3(C-17),42.2(C-18),36.9(C-19),132.7 (C-20),117.5(C-21),36.8(C-22),66.7(C-23),14.3(C-24),17.6(C-25),17.8 (C-26),27.0(C-27),176.2 (C-28),23.3 (C-29),95.8(glc-C-1),73.9(glc-C-2),78.9(glc-C-3),71.1(glc-C-4),77.9(glc-C-5),69.6(glc-C-6),105.2(glc′-C-1),75.5(glc′-C-2),76.5(glc′-C-3),77.6(glc′-C-4),77.2(gl′-C-5),61.5(glc′-C-6),102.5(rham-C-1),72.2(rham-C-2),83.5(rham-C-3),73.0(rham-C-4),70.1(rham-C-5),18.5(rham-C-6),107.3(xyl-C-1),75.7(xyl-C-2),78.4(xyl-C-3),71.1(xyl-C-4),67.3(xyl-C-5)。以上数据与文献[12]报道的数据一致,因此鉴定化合物6为akemisaponins D。
化合物7 白色无定形粉末(甲醇)。ESI-MS m/z 965[M+Na]+。1H-NMR(600 MHz,C5D5N) δ:6.23(1H,d,J=7.8 Hz,glc-H-1),5.89(1H,br s,rham-H-1),5.45(1H,br s,H-12),5.21(1H,br s,H-21),4.99(1H,d,J=7.8 Hz,glc′-H-1),4.17(1H,m,H-23a),3.71(1H,d,J=10.2 Hz,H-23b),1.72(3H,d,J=6.0 Hz,rham-H-6),1.60(3H,s,H-29),1.19(3H,s,H-27),1.16(3H,s,H-26),1.14(3H,s,H-25),1.10(3H,s,H-24);13C-NMR(150 MHz,C5D5N)δ:47.8(C-1),69.0(C-2),78.3(C-3),43.8(C-4),48.1(C-5),18.7(C-6),32.6(C-7),40.2(C-8),48.3(C-9),38.5(C-10),24.1(C-11),123.2(C-12),143.7(C-13),42.2(C-14),28.7(C-15),26.0(C-16),45.2(C-17),42.2(C-18),36.9(C-19),132.8 (C-20),117.5(C-21),36.8(C-22),66.8(C-23),14.4(C-24),17.6(C-25),17.7 (C-26),27.1(C-27),176.3 (C-28),23.3(C-29),95.8(glc-C-1),73.9(glc-C-2),78.4(glc-C-3),71.1(glc-C-4),78.7(glc-C-5),69.6(glc-C-6),105.2(glc′-C-1),75.4(glc′-C-2),76.6(glc′-C-3),78.6(glc′-C-4),77.2(glc′-C-5),61.5(glc′-C-6),102.8(rham-C-1),72.6(rham-C-2),72.8(rham-C-3),74.1(rham-C-4),70.4(rham-C-5),18.5(rham-C-6)。以上数据与文献[12]报道的数据一致,因此鉴定化合物7为akemisaponins E。
化合物8 白色无定形粉末(甲醇)。ESI-MS m/z 957 [M-H]-。1H-NMR(600 MHz,C5D5N)δ:6.20(1H,d,J=7.8 Hz,glc-H-1),5.89(1H,br s,rham-H-1),5.42(1H,br s,H-12),4.99(1H,d,J=7.8 Hz,glc′-H-1),4.18(1H,m,H-23a),3.72(1H,d,J=10.8 Hz,H-23b),1.71(3H,d,J=6.0 Hz,rham-H-6),1.19(3H,s,H-26),1.13(3H,s,H-25),1.08(3H,s,H-27),1.08(3H,s,H-24),0.91(3H,d,J=6.0 Hz,H-29),0.87(3H,d,J=6.0 Hz,H-30);13C-NMR(150 MHz,C5D5N)δ:48.0(C-1),69.0(C-2),78.2(C-3),43.6(C-4),48(C-5),18.5(C-6),33.2(C-7),40.2(C-8),48.1(C-9),38.4(C-10),23.9(C-11),126.0(C-12),138.6(C-13),42.5(C-14),28.7(C-15),24.6(C-16),48.4(C-17),53.2(C-18),39.3(C-19),39.1(C-20),36.8(C-21),30.9(C-22),66.6(C-23),14.4(C-24),17.7(C-25),17.8(C-26),23.8(C-27),176.3(C-28),23.8(C-29),21.2(C-30),95.6(glc-C-1),73.8(glc-C-2),78.9(glc-C-3),71.1(glc-C-4),77.9(glc-C-5),69.5(glc-C-6),105.1(glc′-C-1),75.4(glc′-C-2),78.4(glc′-C-3),77.2(glc′-C-4),77.2(glc′-C-5),61.3(glc′-C-6),102.7(rham-C-1),72.6(rham-C-2),72.8(rham-C-3),74.0(rham-C-4),70.3(rham-C-5),18.4(rham-C-6)。以上数据与文献[13]报道的数据一致,因此鉴定化合物8为积雪草苷。
化合物9 白色无定形粉末(甲醇)。ESI-MS m/z 1 113 [M+Na]+。1H-NMR(600 MHz,C5D5N)δ:6.26(1H,d,J=7.8 Hz,glc-H-1),5.91(1H,br s,rham-H-1),5.42(1H,br s,H-12),5.27(1H,d,J=7.8,1.8 Hz,xyl-H-1),4.97(1H,d,J=7.8 Hz,glc′-H-1),4.22(1H,d,J=10.8 Hz,H-23a),3.72(1H,d,J=10.8 Hz,H-23b),1.69(3H,d,J=6.0 Hz,rham-H-6),1.16(3H,s,H-27),1.16(3H,s,H-26),1.13(3H,s,H-25),1.09(3H,s,H-24),0.88(3H,s,H-29),0.88(3H,s,H-30);13C-NMR(150 MHz,C5D5N)δ:47.7(C-1),68.8(C-2),78.2(C-3),43.6(C-4),47.8(C-5),18.6(C-6),34.0(C-7),39.9(C-8),48.1(C-9),38.3(C-10),23.9(C-11),122.7(C-12),144.0(C-13),42.1(C-14),28.2(C-15),23.3(C-16),46.8(C-17),41.5(C-18),46.1(C-19),30.7(C-20),32.7(C-21),32.4(C-22),66.3(C-23),14.3(C-24),17.4(C-25),17.5 (C-26),25.9(C-27),176.4 (C-28),32.9 (C-29),23.6 (C-30),95.6(glc-C-1),73.8(glc-C-2),78.7(glc-C-3),71(glc-C-4),77.9(glc-C-5),69.2(glc-C-6),105(glc′-C-1),75.3(glc′-C-2),76.3(glc′-C-3),77.1(glc′-C-4),77.1(glc′-C-5),61.2(glc′-C-6),102.4(rham-C-1),72.1(rham-C-2),83.3(rham-C-3),73.0(rham-C-4),70.0(rham-C-5),18.5(rham-C-6),107.4(xyl-C-1),75.7(xyl-C-2),78.3(xyl-C-3),71.1(xyl-C-4),67.3(xyl-C-5)。以上数据与文献[14]报道的数据一致,因此鉴定化合物9为皂苷PJ1。
化合物10 白色无定形粉末(甲醇)。ESI-MS m/z 957[M+Na]+。1H-NMR(600 MHz,C5D5N)δ:6.23(1H,d,J=7.8 Hz,glc-H-1),5.85(1H,br s,rham-H-1),5.38(1H,br s,H-12),5.21(1H,br s,H-21),4.98(1H,d,J=7.8 Hz,glc′-H-1),4.11(1H,m,H-23a),3.65(1H,d,J=10.2 Hz,H-23b),1.69(3H,d,J=6.0 Hz,rham-H-6),1.12(3H,s,H-27),1.12(3H,s,H-26),1.10(3H,s,H-25),1.06(3H,s,H-24),0.85(3H,s,H-29),0.83(3H,s,H-30);13C-NMR(150 MHz,C5D5N)δ:47.8(C-1),69.0(C-2),78.3(C-3),43.8(C-4),48.1(C-5),18.7(C-6),32.6(C-7),40.2(C-8),48.3(C-9),38.5(C-10),24.1(C-11),123.2(C-12),143.7(C-13),42.2(C-14),28.7(C-15),26.0(C-16),45.2(C-17),42.2(C-18),36.9(C-19),132.8(C-20),117.5(C-21),36.8(C-22),66.8(C-23),14.4(C-24),17.6(C-25),17.7(C-26),27.1(C-27),176.3(C-28),33.1(C-29),23.7(C-30),95.8(glc-C-1),73.9(glc-C-2),78.4(glc-C-3),71.1(glc-C-4),78.7(glc-C-5),69.6(glc-C-6),105.2(glc′-C-1),75.4(glc′-C-2),76.6(glc′-C-3),78.6(glc′-C-4),77.2(glc′-C-5),61.5(glc′-C-6),102.8(rham-C-1),72.6(rham-C-2),72.8(rham-C-3),74.1(rham-C-4),70.4(rham-C-5),18.5(rham-C-6)。以上数据与文献[15]报道的数据一致,因此鉴定化合物10为scheffoleoside A。
化合物11 淡黄色粉末(甲醇)。ESI-MS m/z 623[M+Na]+。1H-NMR(600 MHz,CD3OD)δ:6.61(2H,s,H-2′,6′),6.53(2H,s,H-2,6),4.30(1H,d,J=7.8 Hz,glc-H-1),3.84(6H,s,3′,5′-OMe),3.79(6H,s,3,5-OMe)。13C-NMR(150 MHz,CD3OD) δ:139.5(C-1),106.4(C-2),153.9(C-3),135.5(C-4),153.9(C-5),106.4(C-6),73.9(C-7),88.0(C-8),61.8(C-9),139.5(C-1′),106.7(C-2′),154.7(C-3′),135.5(C-4′),154.7(C-5′),106.7(C-6′),33.5(C-7′),35.9(C-8′),62.9(C-9′),105.6(glc-C-1),76.5(glc-C-2),77.9(glc-C-3),71.9(glc-C-4),78.7(glc-C-5),62.9(glc-C-6),56.6(C-3,5-OMe),56.9(C-3′,5′-OMe)。以上数据与文献[16]报道的数据一致,因此鉴定化合物11为symplocosneolignan A。
化合物12 白色无定形粉末(甲醇)。ESI-MS m/z 1 389[M+Na]+。1H-NMR(600 MHz,C5D5N)δ:6.26(1H,d,J=7.8 Hz,glc-H-1),5.87(1H,br s,rham-H-1),5.62(1H,br s,rham′-H-1),5.41(1H,br s,H-12),5.40(1H,d,J=7.8 Hz,glc″-H-1),5.21(1H,br s,H-21),5.01(1H,d,J=7.8 Hz,glc′-H-1),4.73(1H,d,J=7.2 Hz,ara-H-1),3.36(1H,dd,J=4.8,12.0 Hz,H-3),1.71(3H,d,J=6.0 Hz,rham-H-6),1.62(3H,d,J=6.0 Hz,rham′-H-6),1.31(3H,s,H-30),1.26(3H,s,H-29),1.10(3H,s,H-27),1.00(3H,s,H-26),0.90(3H,s,H-25),0.89(3H,s,H-24),0.88(3H,s,H-23)。13C-NMR(150 MHz,C5D5N)δ:39.1(C-1),26.9(C-2),89.2(C-3),40.2(C-4),56.4(C-5),18.9(C-6),32.8(C-7),40.3(C-8),48.3(C-9),37.3(C-10),24.1(C-11),123.2(C-12),144.4(C13),42.4(C-14),28.6(C-15),23.7(C-16),47.3(C-17),42.0(C-18),46.5(C-19),31.1(C-20),34.4(C-21),33.2(C-22),28.3(C-23),17.5(C-24),16.0(C-25),17.9(C-26),26.3(C-27),176.8(C-28),33.4(C-29),24.0(C-30),96.3(glc-C-1),73.2(glc-C-2),79.2(glc-C-3),70.1(glc-C-4),77.7(glc-C-5),68.5(glc-C-6),105.7(glc′-C-1),74.4(glc′-C-2),75.8(glc′-C-3),78.3(glc′-C-4),77.0(glc′-C-5),61.8(glc′-C-6),103.2(rham-C-1),71.2(rham-C-2),73.1(rham-C-3),73.4(rham-C-4),69.9(rham-C-5),19.0(rham-C-6),105.1(ara-C-1),74.9(ara-C-2),83.1(ara-C-3),68.7(ara-C-4),65.5(ara-C-5),104.5(glc″-C-1),74.8(glc″-C-2),78.2(glc″-C-3),71.6(glc″-C-4),78.3(glc″-C-5),62.6(glc″-C-6),101.7(rham′-C-1),72.5(rham′-C-2),72.4(rham′-C-3),73.8(rham′-C-4),70.1(rham′-C-5),18.4(rham′-C-6)。以上数据与文献[17]报道的数据一致,因此鉴定化合物12为刺楸皂苷D。
化合物13 白色无定形粉末(甲醇)。ESI-MS m/z 1 405[M+Na]+。1H-NMR(600 MHz,C5D5N)δ:6.24(1H,d,J=7.8 Hz,glc-H-1),5.86(1H,br s,rham-H-1),5.50(1H,d,J=7.8 Hz,glc′″-H-1),5.41(1H,br s,H-12),5.33(1H,d,J=7.8 Hz,glc″-H-1),5.21(1H,br s,H-21),5.01(1H,d,J=7.8 Hz,glc′-H-1),4.73(1H,d,J=7.2 Hz,ara-H-1),3.36(1H,dd,J=4.8,12.0 Hz,H-3),1.71(3H,d,J=6.0 Hz,rham-H-6),1.30(3H,s,H-30),1.26(3H,s,H-29),1.10(3H,s,H-27),1.00(3H,s,H-26),0.91(3H,s,H-25),0.90(3H,s,H-24),0.89(3H,s,H-23)。13C-NMR(150 MHz,C5D5N)δ:39.1(C-1),26.9(C-2),89.3(C-3),40.2(C-4),56.4(C-5),18.9(C-6),32.8(C-7),40.3(C-8),48.3(C-9),37.3(C-10),24.1(C-11),123.1(C-12),144.4(C-13),42.4(C-14),28.6(C-15),23.7(C-16),47.3(C-17),42.0(C-18),46.5(C-19),31.1(C-20),34.4(C-21),33.2(C-22),28.3(C-23),17.5(C-24),16.0(C-25),17.9(C-26),26.3(C-27),176.9(C-28),33.4(C-29),24.0(C-30),96.5(glc-C-1),73.5(glc-C-2),79.0(glc-C-3),70.0(glc-C-4),77.5(glc-C-5),68.6(glc-C-6),105.7(glc′-C-1),74.4(glc′-C-2),75.8(glc′-C-3),78.3(glc′-C-4),77.0(glc′-C-5),61.8(glc′-C-6),103.2(rham-C-1),71.2(rham-C-2),73.2(rham-C-3),73.4(rham-C-4),69.9(rham-C-5),18.9(rham-C-6),105.2(ara-C-1),77.4(ara-C-2),83.3(ara-C-3),68.8(ara-C-4),65.9(ara-C-5),105.3(glc″-C-1),75.7(glc″-C-2),78.6(glc″-C-3),72.1(glc″-C-4),77.5(glc″-C-5),63.1(glc″-C-6),104.4(glc′″-C-1),76.7(glc′″-C-2),78.3(glc′″-C-3),71.0(glc′″-C-4),78.6(glc′″-C-5),62.7(glc′″-C-6)。以上数据与文献[18]报道的数据一致,因此鉴定化合物13为leonticin E。
化合物14 白色无定形粉末(甲醇)。ESI-MS m/z 1 097 [M+Na]+。1H-NMR(600 MHz,C5D5N)δ:6.26(1H,d,J=7.8 Hz,glc-H-1),5.88(1H,br s,rham-H-1),5.40(1H,br s,H-12),4.96(1H,d,J=7.8 Hz,glc”-H-1),5.21(1H,br s,H-21),5.00(1H,d,J=7.8 Hz,glc′-H-1),4.78(1H,d,J=7.2 Hz,ara-H-1),3.36(1H,dd,J=4.8,12.0 Hz,H-3),1.69(3H,d,J=6.0 Hz,rham-H-6),1.31(3H,s,H-30),1.25(3H,s,H-29),1.11(3H,s,H-27),1.01(3H,s,H-26),0.91(3H,s,H-25),0.89(3H,s,H-24),0.88(3H,s,H-23)。13C-NMR(150 MHz,C5D5N)δ:39.2(C-1),27.4(C-2),89.2(C-3),40.2(C-4),56.4(C-5),18.9(C-6),33.7(C-7),40.3(C-8),48.6(C-9),37.6(C-10),24.4(C-11),123.2(C-12),144.3(C-13),42.4(C-14),28.9(C-15),23.9(C-16),47.5(C-17),41.8(C-18),46.7(C-19),30.6(C-20),34.8(C-21),32.7(C-22),28.8(C-23),17.6(C-24),16.3(C-25),17.9(C-26),26.8(C-27),176.9(C-28),33.7(C-29),24.4(C-30),96.5(glc-C-1),73.5(glc-C-2),79.0(glc-C-3),70.0(glc-C-4),77.5(glc-C-5),68.6(glc-C-6),105.7(glc′-C-1),74.5(glc′-C-2),75.8(glc′-C-3),78.3(glc′-C-4),77.2(glc′-C-5),61.8(glc′-C-6),103.2(rham-C-1),71.2(rham-C-2),73.2(rham-C-3),73.4(rham-C-4),69.9(rham-C-5),18.9(rham-C-6),105.7(ara-C-1),81.1(ara-C-2),83.2(ara-C-3),69.2(ara-C-4),67.5(ara-C-5),105.3(glc″-C-1),75.7(glc″-C-2),76.8(glc″-C-3),79.7(glc″-C-4),77.7(glc″-C-5),61.6(glc″-C-6)。以上数据与文献[19]报道的数据一致,因此鉴定化合物14为ciwujianoside A1。

